Maintenance of Pharmaceutical Clean Area: FDA Recommendation
FDA recommendations on the pharmaceutical clean area and supporting area maintenance in sterile manufacturing unit.
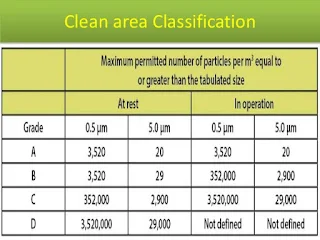
Maintenance of pharmaceutical clean area has its importance in sterile manufacturing. Sterility of the sterile products is the most important factor for the product quality and it can be achieved only by the proper maintenance of the manufacturing area.
Areas surrounding the core manufacturing area are known as supporting areas. These supporting areas have different functions as storage of in- process materials, cleaned equipment, material transfer etc. These areas should be designed to minimize the particulate and microbial contamination in the core manufacturing area where the product is exposed to the air.
Different activities in surrounding areas should be conducted according to the cleanliness class of the area. Less critical activities such as equipment washing should be done in the 1,00,000 class area. The adjacent area to the aseptic area (class 100) should be maintained at least at class 10,000. The manufacturer can maintain it as class 1000 or 100 depending upon the activities done in the pharmaceutical clean area.
Separation of areas used in manufacturing operation is necessary to prevent the contamination. The areas of higher air cleanliness class should have proper airflow and higher pressure differential than the less cleanliness class areas. Rooms maintained at higher cleanliness class should have positive pressure differential than the adjacent rooms.
According to FDA, the pressure differential should be at least 0.05 inch of water. At the opening of door, the air should flow from the higher cleanliness room to lower to prevent the entrance of the contamination. Pressure differential should be maintained throughout the manufacturing process runs and it should be monitored and recorded in every shift as directed by FDA for the pharmaceutical clean area. Any deviation found from the limits must be investigated.
To maintain the clean room area and its air quality it is required to maintain the desired air flow. For the supporting areas of class 1,00,000 airflow should be maintained to get at least 20 air changes per hour. It would be difficult to maintain this area below these air changes.
As per FDA guidance, an automated monitoring system should be established for that detects the critical changes those can alter the area cleanliness. As for differential pressure, low pressure in any of the classified areas should be detected and an alarm should be raised for the same to prevent the entrance of unclassified air into the area.





0 Comments
If you have any doubts, please let me know
Emoji