Personnel Monitoring in Sterile Area
Learn how to monitor the personnel hygene in Sterile Area.
Procedure
Allow solidifying the plates under LAF, after solidification label all the plates with the name of media, preparation batch No. and date of preparation.
Invert and incubate the plates at 30 to 35°C for 24 hrs. After incubation check the plates for any contamination, if there is contamination discard the plates as per SOP for Destruction of Microbial waste by Autoclaving.
After pre-incubation, label all the plates with the date of sampling, Personnel name and Shift with the help of marker pen and wrap with aluminum foil and then keep in a clean stainless steel container.
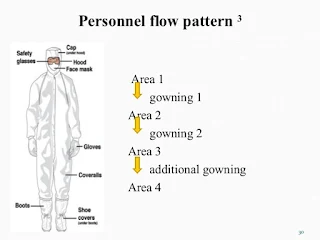
Transfer the container 1st airlock of the material entry of sterile area and personnel must be entered through airlocks (personnel entry) as per SOP for Entry and gowning procedure for the sterile area.
Collect the materials from 1st airlock of the material entry of sterile area and disinfect the container with sterile 70% IPA solution.
Call operator or personnel to be monitored, open the plate and tell personnel to place his/her right-hand fingers with gloves gently on the surface of SCDA plate. Use a fresh plate for left-hand finger and follow the same procedure.
Close the plate and follow the same procedure for minimum two persons.
Prepare a positive control plate by streaking any pure culture of E. coli / Salmonella / Staph. aureus/ Ps. aeruginosa, on the surface of SCDA plate. For negative control, incubate the plate as it is without streaking.
Invert, and incubate all the plates at 20-25°C for 72 hrs and 30 to 35°C for 48 hours.
After incubation, count the number of cfu formed on the plates with the help of colony counter. Operate as per SOP for colony counter and record the results per 5 finger.
Precaution
After finger dab testing, immediately wash the hands with sterile 70% IPA solution.
Result must be expressed per five finger
All pre-incubated plates should be rejected if a single plate shows evidence of microbial contamination.
5.0 Frequencies Daily
6.0 Abbreviation
IPA: Isopropyl Alcohol
Comments
Post a Comment
If you have any doubts, please let me know